

Problems
Our solution
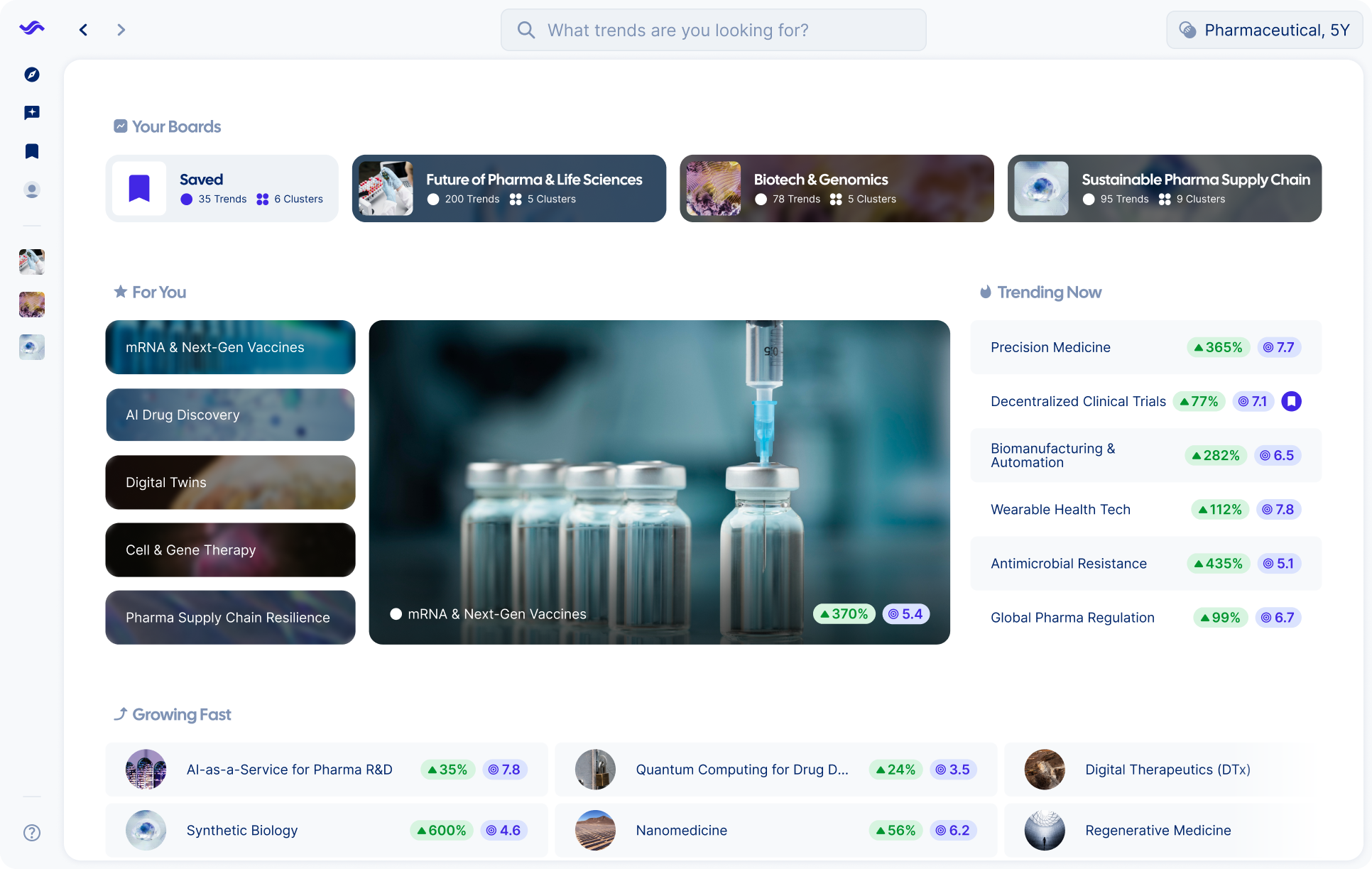

Spot opportunities sooner than anyone else
AI Analyst for pharma leaders
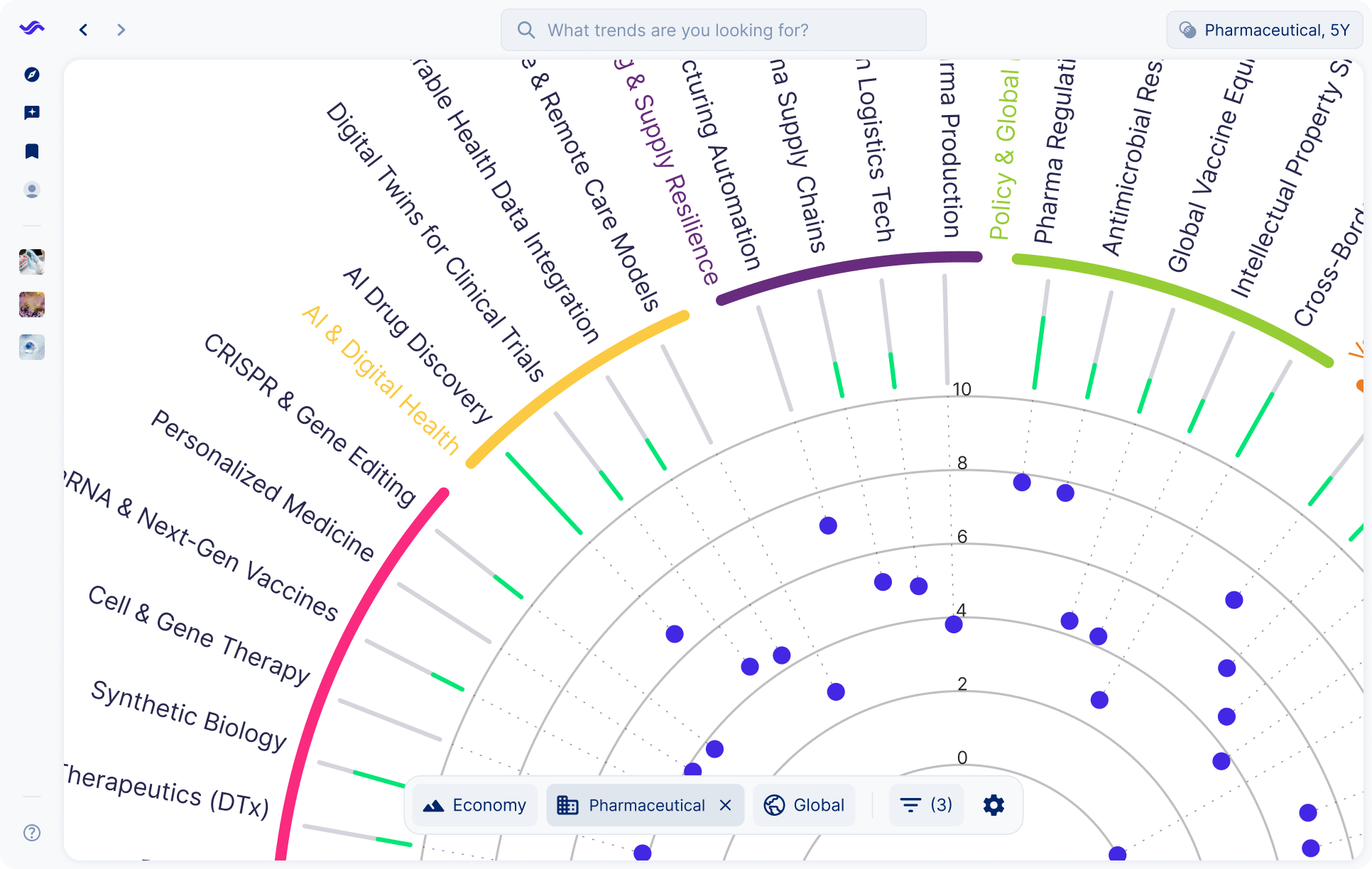
See your global energy landscape clearly
Track trends in real time
Key benefits
Strategists trust Trendtracker to turn data overload into instant, actionable insights.
Trends in the industry


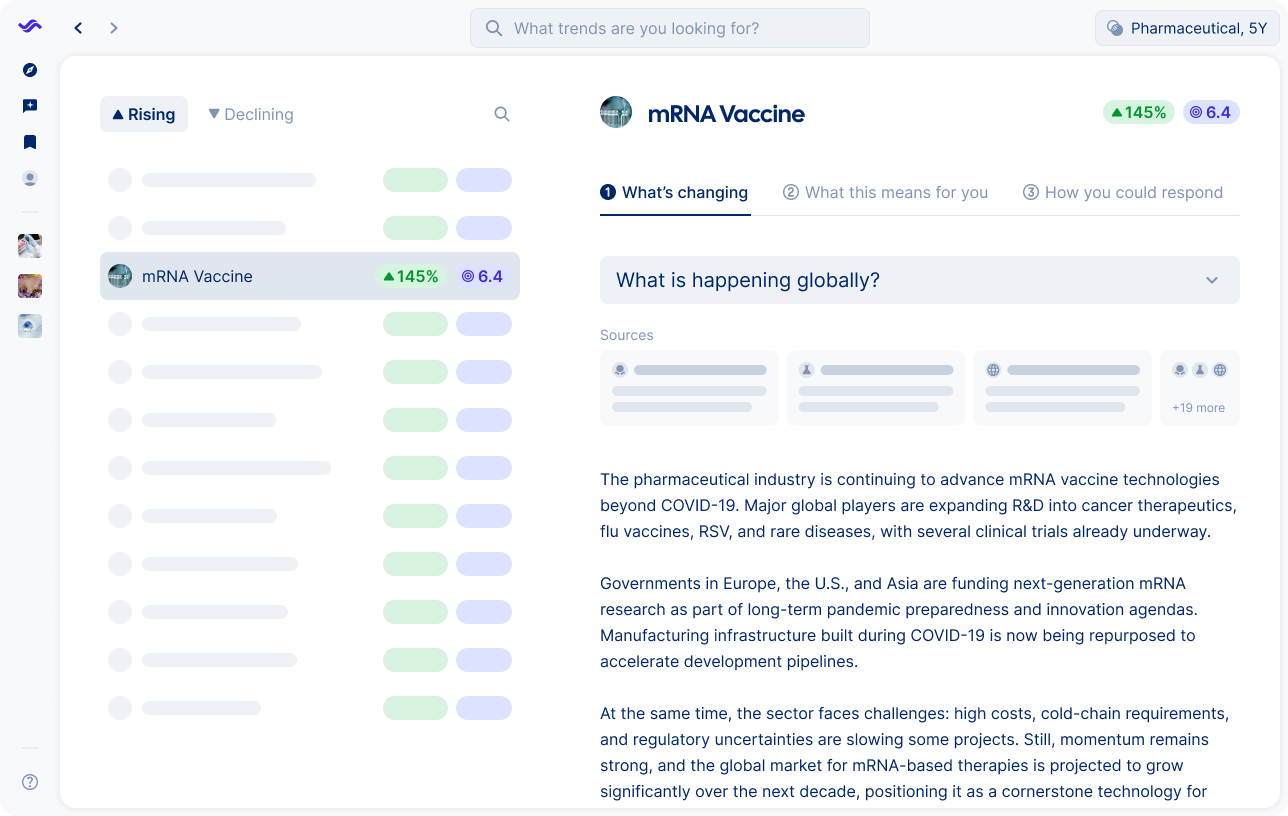
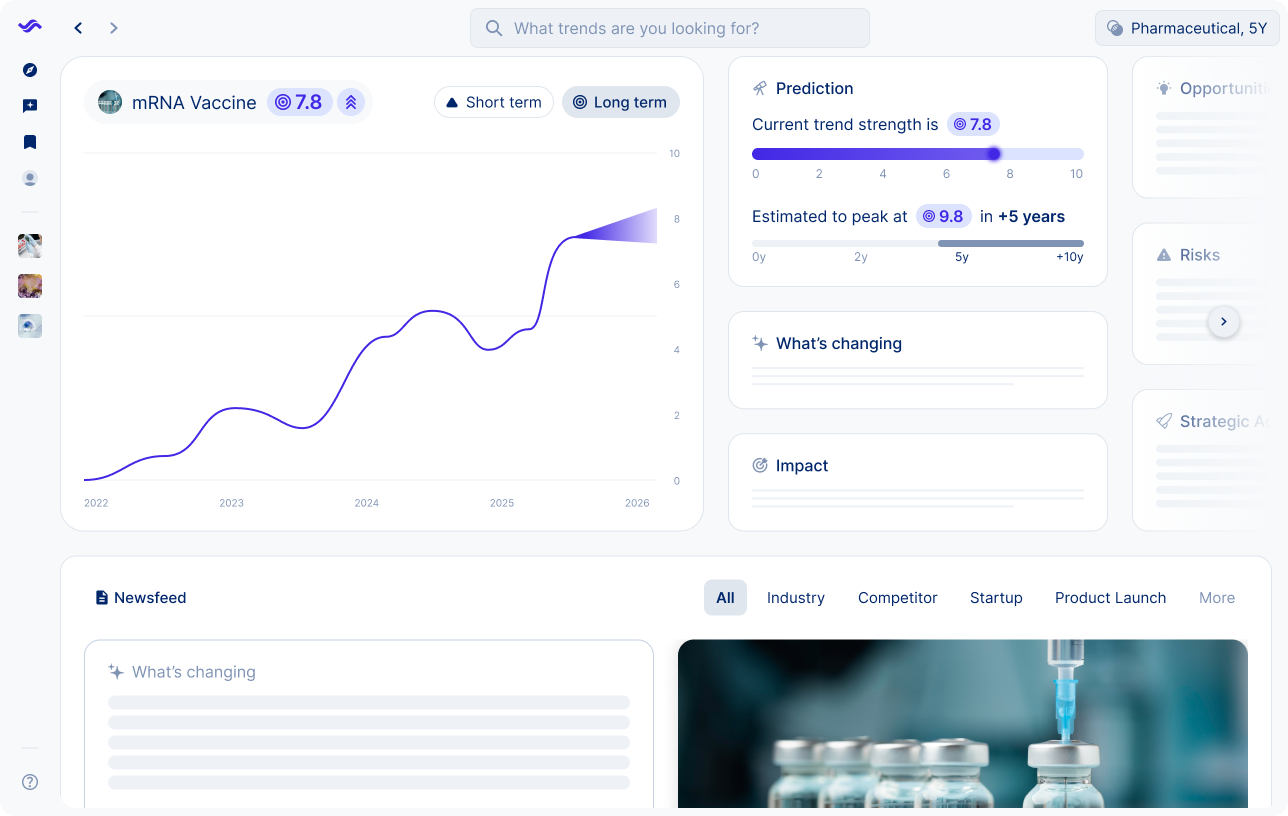
mRNA vaccines are shifting from emergency pandemic tools to broader medical applications. While some projects are paused due to funding cuts and regulatory scrutiny, research continues to show potential in oncology, rare diseases and neurological conditions.
Pharmaceutical companies face a complex environment where public trust, investor confidence and regulatory demands influence progress. mRNA is evolving into a platform technology that could reshape treatment pipelines and redefine competition in the years ahead.
Companies can strengthen their position by deepening research partnerships, expanding applications beyond vaccines and engaging transparently with regulators and patients. At the same time, investment in digital tools and AI can support safety monitoring and compliance, helping to secure long term resilience.


RNA therapeutics are gaining momentum as new partnerships and investments accelerate innovation. Advances in delivery systems and AI-driven drug design are opening possibilities in precision medicine, even though technical and regulatory challenges remain.
Pharma companies see RNA as a pathway to targeted therapies that address unmet medical needs. While delivery hurdles and cost pressures still limit scalability, the growing number of collaborations signals a competitive shift where RNA is becoming a cornerstone of next generation treatment strategies.
Companies can strengthen their position by advancing delivery technologies, forming biotech and research alliances, and investing in digital tools for compliance and safety. A clear focus on precision medicine and patient centric approaches will help translate RNA’s promise into long term market impact.


AI, organoid models and quantum computing are reshaping drug discovery, making it faster, more accurate and cost effective. New business models and research partnerships are driving collaboration across the industry, opening access to innovative therapies worldwide.
Drug discovery is shifting from slow, resource intensive pipelines to data driven approaches that promise quicker results and higher precision. This transformation is creating new competitive pressures while also raising regulatory and ethical questions about how AI and advanced tools are applied.
Pharma companies can accelerate progress by adopting AI powered methods, expanding global research partnerships and investing in sustainable manufacturing. Building transparency with regulators and engaging in open collaboration will be key to turning discovery advances into reliable and trusted treatments.
Drowning in data? See how Trendtracker’s AI turns information overload into instant, actionable clarity for pharma strategists.
The pharmaceutical industry is navigating a wave of disruption. Breakthrough technologies, shifting regulations, and rising public expectations are reshaping how drugs are discovered, developed, and delivered. Companies face pressure to innovate while ensuring resilience in the face of supply chain shocks, pricing debates, and rapid scientific advances.
New scientific frontiers are opening up opportunities for treatments that were unimaginable a decade ago. RNA platforms, cell and gene therapies, and precision medicine are transforming pipelines and challenging traditional pharma business models.
Today’s landscape
mRNA technology proved its value during the pandemic, but research is now moving into oncology, rare diseases, and even neurological conditions. Companies like Moderna and BioNTech are investing heavily in broader applications, while governments and investors weigh the long-term potential.
The road ahead
Expect mRNA to become a versatile therapeutic platform, enabling faster development cycles and more personalized treatments. The challenge will be managing regulatory scrutiny, scaling manufacturing, and securing sustained funding.
PESTLE: Technological (Tech), Political/Legal (P/L), Economic (Econ), Environmental (Env)
Horizon: Mid-term (5–10y)
Strategic action: Build partnerships with biotech innovators, diversify R&D portfolios, and engage regulators early to accelerate approvals.
Today’s landscape
RNA therapeutics are gaining traction as advances in delivery systems make treatments more viable. These therapies are opening new possibilities for conditions with unmet medical needs, from genetic disorders to metabolic diseases.
The road ahead
RNA will expand beyond vaccines into a class of therapies central to pharma pipelines. Breakthroughs in delivery and scalability will determine which companies capture market leadership.
PESTLE: Technological (Tech), Economic (Econ), Political/Legal (P/L)
Horizon: Mid- to long-term (5–15y)
Strategic action: Invest in delivery technologies, form alliances with research institutes, and prioritize patient-centric applications where RNA offers unique advantages.
Today’s landscape
Genomics, AI, and diagnostic tools are making personalized medicine more accessible. Pharma is moving beyond “one-size-fits-all” drugs toward treatments tailored to individual patient profiles, especially in oncology.
The road ahead
Personalized medicine will increasingly shape market expectations, with payers and regulators demanding proven outcomes. This will require new business models around pricing, access, and data sharing.
PESTLE: Social (S), Technological (Tech), Economic (Econ), Political/Legal (P/L)
Horizon: Near- to mid-term (0–7y)
Strategic action: Develop robust data ecosystems, partner with diagnostic companies, and explore outcome-based pricing models to align incentives.



Trials are moving beyond hospital walls. Digital tools, new endpoints, and richer real-world evidence are speeding decisions and widening access. The winners will reduce cycle time while improving data quality and patient experience.
Today’s landscape
Hybrid and remote designs are expanding access and keeping studies on track. Regulators have issued guidance, but execution quality varies.
The road ahead
Expect standard toolkits for eConsent, remote monitoring, and home health support. Site roles evolve as sponsors build direct patient relationships.
PESTLE: Technological, Social, Political/Legal
Horizon: Near- to mid-term (0–5 years)
Strategic action: Create playbooks by therapy area. Invest in patient support logistics and data integrity controls. Engage regulators early on protocol design and data handling.
Today’s landscape
Health records, registries, and claims data are informing protocol design and post-market commitments. Quality and bias remain concerns.
The road ahead
RWE will guide indication expansion and payer negotiations. Methods and audit trails for data provenance become non-negotiable.
PESTLE: Political/Legal, Technological, Economic
Horizon: Mid-term (3–7 years)
Strategic action: Stand up RWE partnerships with providers and payers. Build transparent evidence frameworks and align early with HTA bodies on endpoints.
Today’s landscape
Wearables and sensors are capturing continuous data for neurology, cardiology, and metabolic disorders. Signal validation is the hurdle.
The road ahead
Validated digital endpoints unlock smaller trials, earlier reads, and more patient-centric designs.
PESTLE: Technological, Legal, Social
Horizon: Near- to mid-term (0–6 years)
Strategic action: Co-develop endpoints with device partners and academia. Run validation studies alongside pivotal trials. Build privacy-first architectures and patient communication plans.

Global shocks have exposed vulnerabilities in pharma’s supply chains, from raw materials to final distribution. At the same time, geopolitical tensions and pricing pressures are pushing companies to rethink resilience. Building secure, flexible, and transparent supply networks is now a strategic priority.
Today’s landscape
COVID-19 and geopolitical tensions highlighted overreliance on a handful of regions for active pharmaceutical ingredients (APIs). Companies are moving to diversify sources and localize production.
The road ahead
Expect reshoring and nearshoring to accelerate, with governments incentivizing local capacity. Dual sourcing becomes the new standard.
PESTLE: Political/Legal, Economic, Environmental
Horizon: Near- to mid-term (0–7 years)
Strategic action: Map critical dependencies, establish dual sourcing strategies, and collaborate with governments to secure incentives for local manufacturing.
Today’s landscape
Continuous manufacturing and 3D printing of drugs are emerging as solutions to reduce bottlenecks and scale production quickly. Adoption is still limited but gaining attention.
The road ahead
Advanced manufacturing will enable smaller, more flexible facilities closer to patients. This will reduce risk and speed delivery.
PESTLE: Technological, Economic, Political/Legal
Horizon: Mid-term (5–10 years)
Strategic action: Pilot advanced manufacturing technologies, build modular facilities, and engage regulators to align on new approval processes.
Today’s landscape
Governments and payers are intensifying scrutiny on drug prices, especially in the US and Europe. Patients are demanding fairer access to life-saving therapies.
The road ahead
Expect stricter pricing regulation and outcome-based models. Companies will need to balance innovation with affordability to maintain trust.
PESTLE: Political/Legal, Social, Economic
Horizon: Near- to mid-term (0–6 years)
Strategic action: Develop outcome-based pricing strategies, increase transparency in cost structures, and engage with patient groups to build credibility.
